Qingdao Globalstar Glass Co., Ltd.
Office address: No.6 Shandong Road, Qingdao,China
Factory address : No.656 Yuhai Road,Chengyang District,Qingdao,China.
View More >Analysis of Factors Influencing Delamination in Laminated Glass(1)
August 28, 2024Introduction
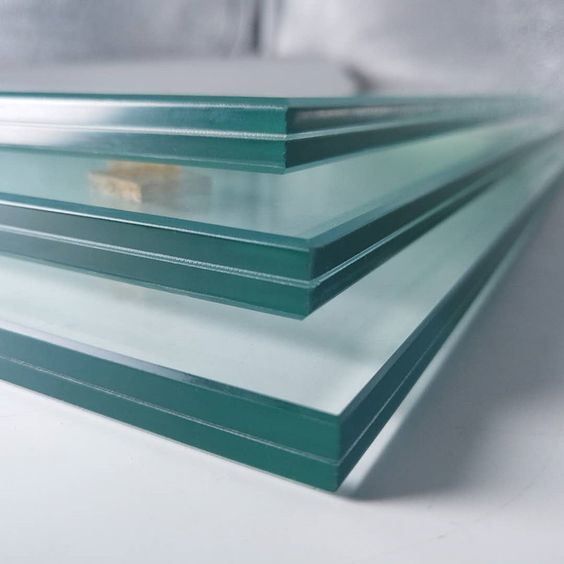
Glass components are highly favored in the architectural field for their unique transparency and aesthetic appeal, with a history of use spanning nearly a thousand years. However, due to the inherent brittleness of glass, the application of single-pane glass in architecture has been significantly limited. In recent decades, the advent of laminated glass and the improvement of lamination technology have greatly expanded the usage and application scope of glass in architecture. Laminated glass consists of two or more layers of glass bonded together by an interlayer film with strong adhesive properties, forming a composite unit that shares the load. This not only enhances the load-bearing capacity of the laminated glass before it fractures but also provides a certain level of post-fracture load-bearing capacity. The adhesive performance between the interlayer film and the glass sheets is a critical factor influencing the overall performance of laminated glass. When delamination occurs in laminated glass, it reduces transparency and affects visual aesthetics; functionally, it impairs or eliminates the interlayer shear transfer, decreasing the overall load-bearing capacity of the laminated glass and compromising its service safety. After the glass fractures, delamination reduces the adhesion of the film to the glass fragments, increasing the likelihood of fragment splatter and potentially leading to the entire glass component falling or collapsing.
There are three primary types of adhesive failure in laminated glass: substrate failure (failure of the glass itself), cohesive failure (failure within the interlayer film), and adhesive failure (separation between the glass and the interlayer film). When external forces exceed the adhesive strength between the film and the glass, separation occurs, manifesting as bubbles, edge warping, or whitening along the edges—commonly referred to as delamination.
Delamination directly impacts the safety, reliability, and durability of laminated glass. The factors influencing this phenomenon are present throughout the entire lifecycle of the glass, from raw material selection and deep processing to maintenance during use. Critical factors include the flatness of the glass sheets, the adhesive properties of the interlayer film, and the types of physical and chemical adsorption at the film-glass interface, all of which directly affect the interlayer adhesion performance.
Researchers like Ou Yangchun have studied how the characteristics of glass sheets, film surfaces, and interfaces influence interlayer adhesion, using adhesive thermodynamics as a basis. They offered recommendations for improving interlayer adhesion through better raw material processing techniques. Ma Qiyuan and Geng Ping explored the effects of the physical and chemical properties of the interlayer film on adhesion and suggested edge sealing treatments for polyvinyl butyral (PVB) laminated glass during its use.
The deep processing of laminated glass is also crucial for ensuring strong adhesion at the film-glass interface. Improper handling during this process can significantly weaken adhesion, leading to defects such as bubbles or edge warping. Environmental factors, including temperature, humidity, UV radiation, exposure to cleaning agents, and the dissolution or corrosion of edge sealants, can further impact the adhesion at the film-glass interface.
Researchers like Wan Detian and Ou Yangchun have used cross-peel tests to measure adhesion strength at the PVB film-glass interface. They also examined how factors such as PVB film thickness, loading speed, UV aging, and humidity aging influence adhesion performance.
Most existing research on laminated glass delamination focuses on isolated factors, such as raw material selection or environmental conditions during use. However, the factors influencing delamination extend across the entire lifecycle of laminated glass, from raw material selection and deep processing to maintenance. A comprehensive understanding of these factors is crucial for effectively preventing and reducing delamination. This paper aims to provide insights into the delamination mechanism of laminated glass by examining the factors at each stage—raw material selection, deep processing, and maintenance—to offer a valuable reference for preventing and mitigating delamination in laminated glass.

1. Analysis of the Bonding Mechanism at the Glass-Film Interface
Laminated glass is a composite material formed by bonding glass with a transparent interlayer film through high temperature and pressure in an autoclave (temperature range 135–140°C, pressure below 1.2 MPa). This process creates a strong bond at the glass-film interface. The adhesion between the film and the glass results from a combination of multiple forces. Depending on the characteristics of the interface, the bonding mechanism primarily includes adsorption forces, chemical bonding, and mechanical interlocking.
Van der Waals forces are the most prevalent adsorption forces at the glass-PVB interface, including interactions like Lewis acid-base forces and hydrogen bonding. Under high-temperature melting conditions, the molecules at the glass-film interface come into close contact and interpenetrate, rearranging themselves under molecular attraction to form physical adsorption forces. Simultaneously, certain polar groups (such as C—OH and Si—OH) interact to form covalent bonds. These combined bonding forces constitute the adhesive strength at the glass-film interface and become "frozen" during the cooling process.
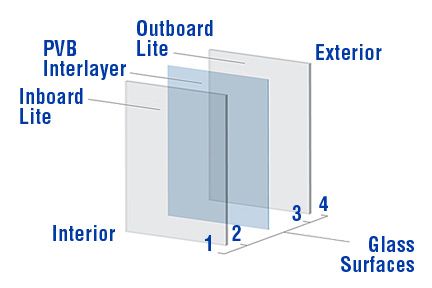
The adhesion between PVB film and the glass surface primarily arises from hydrogen bonds formed between the C—OH groups in the PVB film and the abundant Si—OH groups on the inorganic glass surface. Additionally, some films contain ions or functional groups that can chemically react with the glass surface, forming new ionic bonds that enhance the adhesion at the glass-film interface. For example, the ionic film (SG) contains 1% sodium ions in the copolymer of ethylene and methacrylic acid, which can form ionic bonds with the glass.
Moreover, the glass surface at the microscopic level features cracks, steps, and pores. During the molten state, the film material flows into these micro-defects on the glass surface, increasing the contact area and providing additional mechanical interlocking once the material solidifies.
The bonding performance between glass and the interlayer film depends on the interactions between different molecules at the interface. Ideally, strong physico-chemical forces would form at the interface, ensuring robust adhesion. However, in practice, the conditions necessary for optimal physico-chemical reactions at the interface are often not fully met, resulting in suboptimal bonding performance. Additionally, low-energy impurities adsorbed at the interface can replace the original high-energy surfaces, creating lower-energy surfaces that further degrade the adhesive performance of the interface.
The article comes from Shanghai Jiao Tong University,Zhao chenjun,Yangjian, and so on.